Table of Contents
Fluorite is also called fluor. A common mineral in nature, can be associated with a variety of other minerals, is produced in many parts of the world, there are 5 effective varieties. Equiaxed crystal system, the main component is calcium fluoride (CaF₂).
Crystallized into octahedrons and cubes. Crystal glass luster, bright color, brittle, Mohs hardness of 4, melting point 1360℃, with complete cleavage properties.
Some fluorite can glow under heat from friction or ultraviolet light. Due to this property, it has been called the most colorful gemstone in the world.

Composition: CaF₂, which contains fluorine 48.67%, calcium 51.33%, sometimes contain rare elements; often associated with quartz, calcite, barite and metallides.
Color: Usually yellow, green, blue, purple and other colors, with glass.
Properties: Mohs hardness 4, density 3.18g/cm3, melting point 1360℃, soluble in acid.
Types: quartz-fluorite type, calcite – fluorite type, carbonate – fluorite type, sulphide – fluorite type, barite – fluorite type, siliceous – fluorite type.
Most of the crystals of fluorite are octahedral and cube, rarely dodecahedral crystals. There are also composite crystals made of intersecting octahedrons and cubes.
Cleavage marks are present in most crystals, and cleavage blocks that peel off larger crystals are also common.
Under octahedral crystallization, the cleavage blocks are flat and triangular. The cleavage blocks of cubic crystals are flat cuboids.
The crystals of fluorite often appear interspersed twin-crystals, that is, the phenomenon of two crystals interpenetrating each other. There are also clusters of symbiotic cubic crystals, which may be granulated, grape-like, globular, or irregularly large.
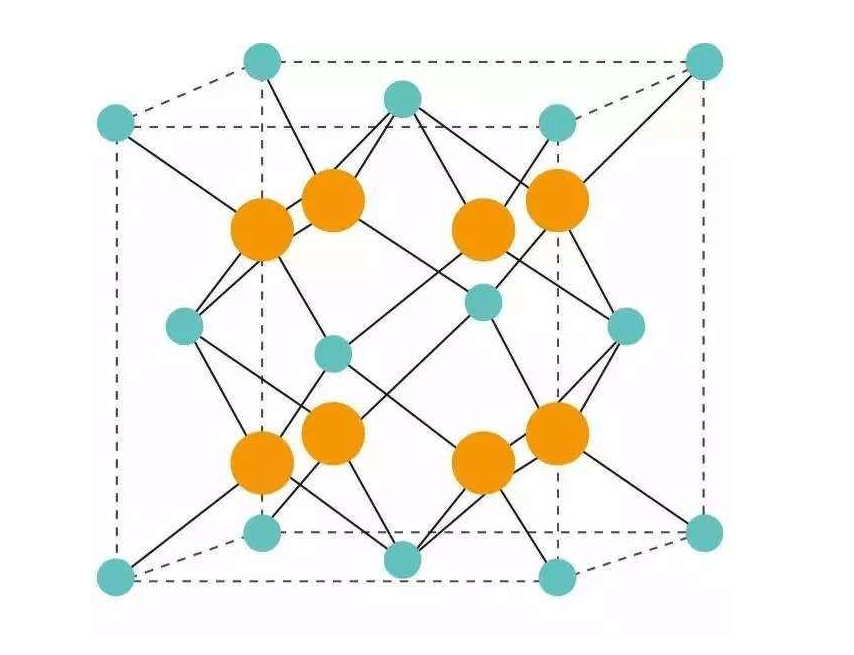
The crystal structure of fluorite is a cubic crystal system. This structure is based on a face-centered dense stack formed by cations, and the tetrahedral spaces are filled by anions.
The Ca2+ ion is located at the junction of the vertical center with a Ca2+ coordination number of 8. The F- ion is located at the center of the eight small cubes within the cube, and F- has a coordination number of 4.

Fluorite is widely used in metallurgy, aluminum smelting, glass, ceramics, cement, chemical industry.
Pure, colorless and transparent fluorite can be used as optical materials, beautiful color fluorite can also be used as gem stone and arts and crafts carving raw materials.
Fluorite is the basic raw material of fluorine chemical industry, its products are widely used in aerospace, aviation, refrigeration, medicine, pesticides, anti-corrosion, fire fighting, electronics, electric power, machinery and atomic energy and other fields.
We can provide you with a variety of crushing, grinding fluorite equipment, if you have needs can contact us.